The securPharm-system
The securPharm system is based on the end-to-end principle during which both ends of the supply chain help ensure safety. The one end is the marketing authorisation holder who markets pharmaceuticals. The other end represents the place where pharmaceuticals are dispensed, i.e. a community pharmacy.
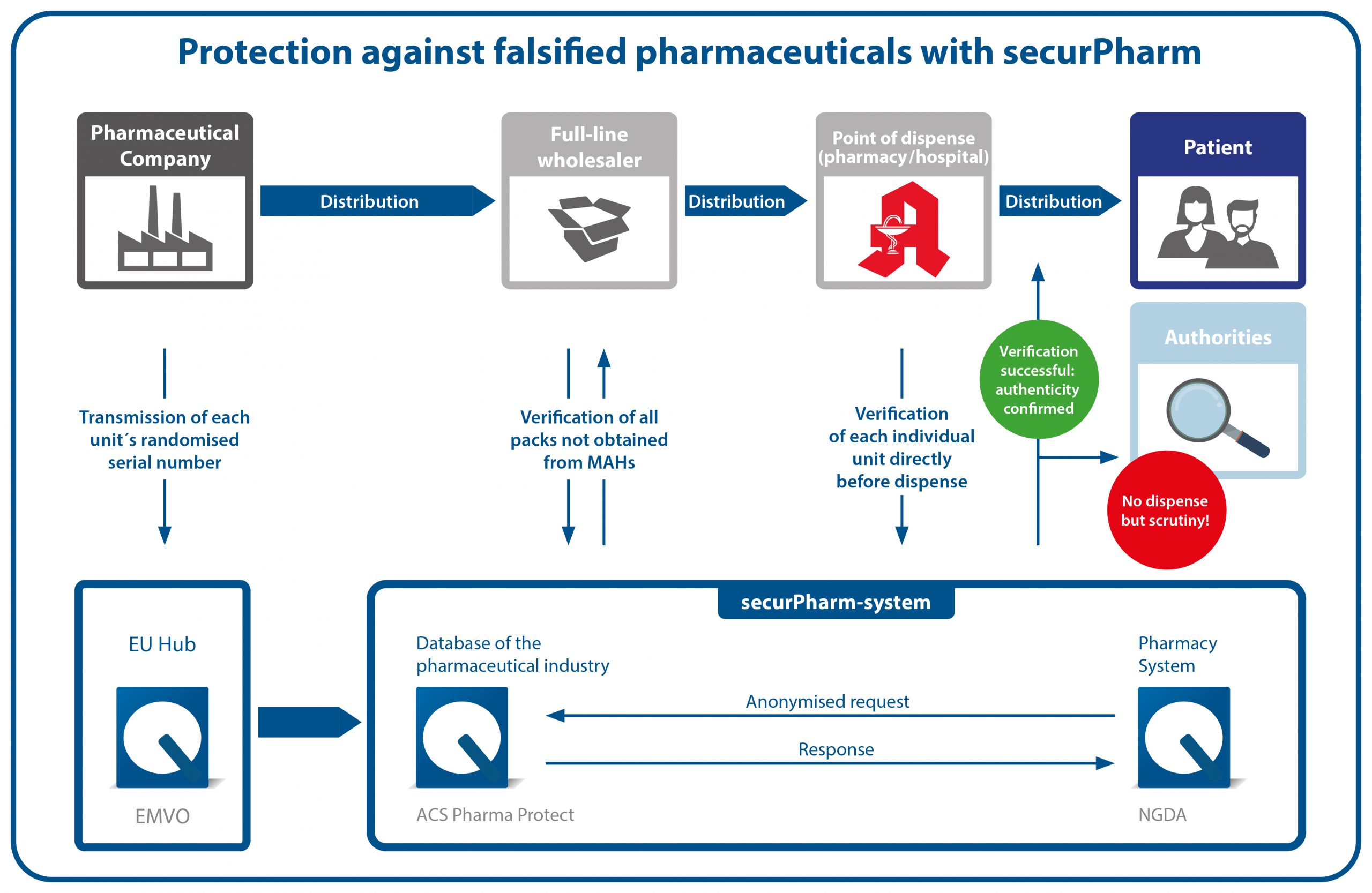
The German verification system is embedded in a European network in order to also safeguard patient protection across national borders. The EU hub, which is operated by the European Medicines Verification Organisation (EMVO) facilitates the data exchange between the EU, the EEA and Switzerland.
Data privacy
For data privacy reasons, the securPharm system works with separate systems for pharmaceutical companies (database system of the pharmaceutical industry) and the places dispensing pharmaceuticals (pharmacy system). Verification inquiries are bundled via the pharmacy system and directed to the database system of the pharmaceutical industry in anonymised form.
Verification process
During the production process, the marketing authorisation holder affixes the safety features to each pharmaceutical package. The data of the unique identifier (serial number, product code, batch number, expiry date) are applied to the pack in clear text and in the form of the Data Matrix Code and uploaded to the centralised database of the pharmaceutical industry. Before the pharmaceutical is dispensed to the patient, the Data Matrix Code is scanned for authentication, thereby reconciling the pack data with the data in the system. The package status is reported back to the pharmacy, i.e. whether the unique identifier is active or has already been deactivated. If the latter is the case (e.g. because the package with this identifier was already dispensed), the pack must not be dispensed to the patient. The incident will subsequently be investigated. If the identifier is active, the pharmaceutical can be dispensed.
How does the system provide protection from falsified medicines?
The European protective system makes falsified medicines in the legal supply chain more easily recognizable, since each serial number can only be used once. Furthermore, the serial number in combination with the product code and the batch number also allows tracing back where a falsified medicine entered the legal supply chain. In the case of stolen goods, the serial number can be locked in the system so that the merchandise can no longer be dispensed.



